
Intracranial Pressure (ICP) is measured in millimeters of mercury (mmHg). Most scholars agree that on average:
- Intracranial pressures (ICP) between 5-15 mmHg, are considered Normal pressure
- Intracranial pressures (ICP) between 20-30 mmHg, are considered Mild to moderate intracranial hypertension which “requires treatment in most circumstances”,
- Intracranial pressures (ICP) of > 40 mmHg indicates “Severe and possibly life-threatening intracranial hypertension.” [1]
When high intracranial pressure is left untreated, it creates a “pushing effect” towards the only natural escape at the base of the skull (the foramen magnum), and the cerebellar tonsils in the pathway are pushed through the foramen magnum. [2]
Understanding the Monro-Kellie Doctrine (the pressure-volume relationship)
The association between IH/IIH and Chiari Malformation appears to be a malicious intricate pathological circle. The cranium (skull) consists of brain matter, cerebrospinal fluid, and both venous and arterial blood. A hypothesis, referred to as the Monro-Kellie Hypothesis (now better known as the Monro-Kellie Doctrine), states, “The sum of volumes of the brain, CSF, and intracranial blood is constant. An increase in one should cause a decrease in one or both of the remaining two.” Therefore, if an abundance of cerebrospinal fluid (IIH or hydrocephalus), both cranial blood volume and brain matter should be forced to deplete. This depletion is usually directed in the path of least resistance – through the foramen magnum and into the spinal canal. When the brain matter closest to the bottom of the skull (cerebellar tonsils) is pushed through the foramen magnum and into the spinal canal (an Acquired Chiari Malformation), the tonsils act like a cork and blocks the flow of cerebrospinal fluid (regardless of the size of the tonsillar descent), which in turn, continues to raise intracranial pressure.[3]
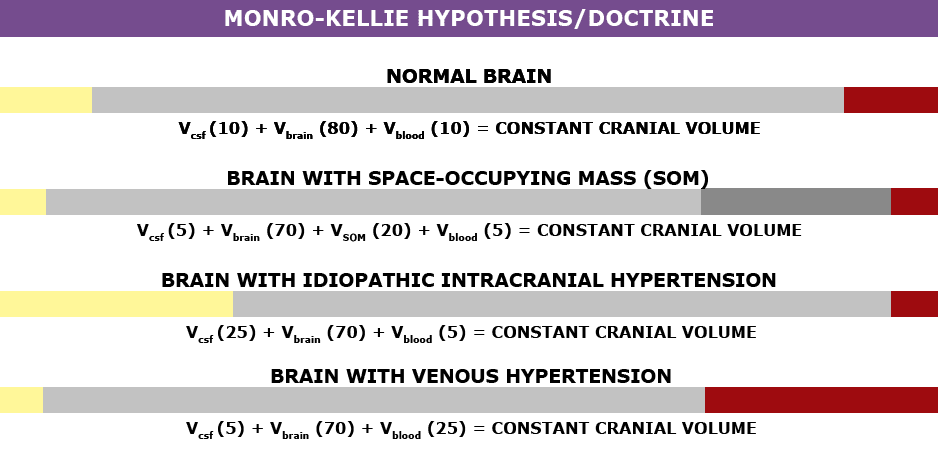
Venous Hypertension
When an etiological cofactor exists (such as a space-occupying mass), it is considered Secondary Intracranial Hypertension (SIH); when no other cause was identified, it is known as Idiopathic Intracranial Hypertension (IIH) formerly known as Pseudotumor Cerebri. However, recent studies on the connection between Intracranial Hypertension and Venous Hypertension might put an end to the “idiopathic” theory.
Oxygen-rich blood travels from the heart to the rest of the body through the arterial system, then the oxygen-depleted blood returns to the heart through the venous system. We have a host of small veins in our head and they dump into a series of large veins, called sinuses.
Dural Venous Sinus Stenosis occurs when there is a narrowing of one or more of the venous sinuses (most commonly seen in the transverse sinuses or transverse/sigmoid sinus junction, but can also occur anywhere within these sinuses or in the jugular). This narrowing compromises cerebral venous outflow through the jugular vein (stenosis/compression of the jugular vein can also result in elevated intracranial pressure [4]). Transverse Sinus Stenosis (TSS) is most common what was initially assumed to be “Idiopathic Intracranial Hypertension (IIH), formerly known as Pseudotumor Cerebri (PTC). Depending on the study that you are reading, it is proving to be present in 90-100% of IIH patients [5]. While its connection might sound obscure, if you look at it from a Monro-Kellie perspective – the blood going into the head, cannot get out at the same speed (because of the narrowed sinus). When this inflow of blood remains constant and the outflow is hindered, the transverse sinus on that side (we have two transverse sinuses, one on each side) enlarges, forcing the CSF and brain matter to reduce to maintain the volume equilibrium. This reciprocation can happen when any of the sinuses or jugular narrow (stenosis). While scholars continue to debate whether TSS is a cause or consequence of IIH, surgeons continue to decompress us without checking our pressures or decompress (the most invasive treatment) in hopes that it will lower our pressures, and patients are left with untreated high pressure still causing a “pushing down effect” and an enlarged foramen magnum for our brains to be pushed down. [2] The sagging brain once again obstructs the flow of cerebrospinal fluid by plugging the foramen magnum, and that in turn raises the intracranial pressure even more. Or, the untreated high pressure blows through the duraplasty and causes a post-operative leak, known as a pseudomeningocele.
Reducing the Risks of Post-Op IH/IIH Complications by Identifying Potential Problems Early:
Brain MRIs often show indicators of Intracranial Hypertension (IH/IIH), therefore, we recommend that all Chiari patients have full brain MRIs and not just cervical MRIs.
- When the pressure builds inside of the dura mater the pressure pushes the dura and fluid inside of the crevice that holds the pituitary gland (the sella turcica or pituitary fossa). When the amount of fluid is equal to or greater than 50% and the pituitary gland size is 2mm, the condition is known as Empty Sella Syndrome. (Doctors now recognize that < 50% (where the pituitary gland size is 3-7mm) can also cause symptoms and they now refer to that as a partially empty sella.) [8]

- Slit like or flattened lateral ventricles from the increased pressure, however, when the Foramen of Monro (the aqueduct that connects the lateral ventricle to the third ventricle) is stenosed, the fluid will back-up and the lateral ventricle will not appear flattened. [7]
- Enlarged/swollen optical nerves (papilledema). [8]
- Low lying or herniated tonsils (often diagnosed as a Chiari Malformation). [2]
Ideas BEFORE DECOMPRESSION is considered:
If you have symptoms of IH/IIH accompanied by any of the MRI indicators mentioned above, it is both reasonable and prudent to ask your neurosurgeon to investigate further BEFORE DECOMPRESSION.
- See a neuro-ophthalmologist to check for signs of papilledema, including Optical Coherence Tomography and Ultrasonographic B-scanning. [8]
- Magnetic Resonance Venography (MRV, preferably with the ATECO technique) to check for venous stenosis of any of the cranial sinuses and/or jugular vein. Stenosis is not exclusive to the transverse sinus and it can happen in multiple sinuses simultaneously.
- If overweight, consider trying to lose weight. Studies show that a weight loss of 5-10% of one’s overall body weight, when accompanied by a low-salt diet, can offer some to IH/IIH symptoms.[9]
- Consider trying Diamox (Acetazolamide) and/or Topamax (Topiramate) to see if that improves the pressure headaches.
- Request a lumbar puncture (spinal tap) to test your opening pressures. We recommend that it’s guided with fluoroscopy with a small gauge needle (and not the standard 22 gauge) that they allow to drip (as opposed to syringe pull) and ensure that someone is available to perform an epidural blood patch if necessary. Time should be allotted afterward to lay flat for several hours immediately following the procedure and for several days once returning home. The potential for CSF leaks is high for the EDS/Chiari patient. A doctor that marginalizes the risks ahead of time, will generally marginalize your symptoms when you are actively leaking.
- ICP Bolt Monitoring can record the differences experienced in pressure over time, and how different positions affect ICP.
Note: When the intracranial pressure gets high enough, it can cause a cranial leak. This is especially true for the Ehlers-Danlos patient where the dura mater is thin and fragile. When a cranial leak decreases the intracranial pressure, the papilledema, empty sella, stenosis, and high-pressure headaches can sometimes start to revert to normal or near-normal, and the leak will affect any attempts to check intracranial pressure (reducing the pressure from what it was before the leak occurred), however, the tonsillar herniation will usually remain if the pressure gets too low. [10]
TREATMENT OPTIONS:
- If you have IH/IIH, ask your doctor to help you reduce the ICP before decompression! This can be done medicinally using Topamax or Diamox. If that doesn’t work, a shunt or stent should be considered.
- A Ventriculoperitoneal Shunt(VP Shunt) or Ventriculoatrial Shunt (VA Shunt) can be surgically placed to drain cerebrospinal fluid straight from the ventricle. Shunts are known for failing and often need a multitude of revisions, but even with all the revisions, it can be less invasive than a decompression. Shunts under the foramen magnum should never be used as a means of controlling ICP.
- An MRV is recommended for all IH/IIH patients. If it indicates venous stenosis, stenting should be considered, as leaving the sinus/jugular stenosed can post other health risks, and stenting can be successful for many with fewer complications that often require revisions.
For the IH/IIH patient, herniated tonsils should be assumed an Acquired Chiari Malformation (even if a small posterior fossa is evident), and by correcting the high pressure before decompression, the decompression will be less likely to fail.
Helpful Tips:
If you have IH/IIH, it is best to avoid caffeine, avoid progestin based birth control, and all EDS patients should try to avoid the use of fluoroquinolones such as ciprofloxacin (Cipro), levofloxacin (Levaquin/Quixin), gatifloxacin (Tequin), moxifloxacin (Avelox), ofloxacin (Ocuflox/Floxin/Floxacin), norfloxacin (Noroxin), due to the increased risk of aneurysm.
References:
1 Rangel-Castillo, Leonardo, et al. “Management of Intracranial Hypertension.” Rangel-Castilla, Leonardo et al. “Management of intracranial hypertension.” Neurologic clinics vol. 26,2 (2008): 521-41, x. doi:10.1016/j.ncl. Feb. 2008, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2452989/>.
2 Aiken, A.H., et al. “Incidence of Cerebellar Tonsillar Ectopia in Idiopathic Intracranial Hypertension: A Mimic of the Chiari I Malformation.” American Journal of Neuroradiology; Nov. 2012, <http://www.ajnr.org/content/33/10/1901>.
3 Mokri, B. “The Monro-Kellie Hypothesis: Applications in CSF Volume Depletion.” Neurology., U.S. National Library of Medicine, 26 June 2001, <https://www.ncbi.nlm.nih.gov/pubmed/11425944>.
4 Zhou, D., et al. “Intracranial hypertension induced by internal jugular vein stenosis can be resolved by stenting.” European Journal of Neurology, November 2017 <https://onlinelibrary.wiley.com/doi/abs/10.1111/ene.13512>.
5 Henderson, Fraser C., et al. “Neurological and Spinal Manifestations of the Ehlers–Danlos Syndromes.” American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 21 Feb. 2017, <www.onlinelibrary.wiley.com/doi/10.1002/ajmg.c.31549/full>.
6 Pietrangelo, Ann. “Empty Sella Syndrome.” Healthline, Oct. 2017, <https://www.healthline.com/health/empty-sella-syndrome>.
7 Hingwala, Divyata R., et al. “Imaging signs in idiopathic intracranial hypertension: Are these signs seen in secondary intracranial hypertension too?.” Annals of Indian Academy of Neurology vol. 16,2: 229-33. doi:10.4103/0972-2327.112476, June 2013, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3724081/>.
8 Mollan, Susan P., et al. “A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension.” Practical neurology vol. 14,6: 380-90. doi:10.1136/practneurol-2014-000821. May 2014, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4251443/>.
9 Thurtell, Matthew J., and Michael Wall. “Idiopathic Intracranial Hypertension (Pseudotumor Cerebri): Recognition, Treatment, and Ongoing Management.” Current Treatment Options in Neurology, U.S. National Library of Medicine, Feb. 2013, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3554852/>.
10 Pérez, Mario A., et al. “Primary spontaneous cerebrospinal fluid leaks and idiopathic intracranial hypertension.” Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society vol. 33,4: 330-7. doi:10.1097/WNO.0b013e318299c292, Dec. 2014, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4040082/>.

![Brain Under Pressure – Understanding Intracranial Hypertension [UPDATED]](https://chiaribridges.org/wp-content/uploads/2021/06/IH_AS271511956_resized.jpg)
![Brain Under Pressure – A Guide to Understanding Intracranial Hypertension [Achive #2]](https://chiaribridges.org/wp-content/uploads/2019/12/Woman_Up-with-headache_AS177290930.jpg)


![The Important Questions to Ask Your Neurosurgeon [Revised]](https://chiaribridges.org/wp-content/uploads/2023/09/MRI-doctor_AS505903501.jpg)
![Brain Under Pressure – Understanding Intracranial Hypertension [Archived]](https://chiaribridges.org/wp-content/uploads/2017/12/Fotolia_87985277_M.jpg)
 Intracranial Hypertension (IH) means high pressure inside the skull. Intracranial Pressure (ICP) is measured in millimeters of mercury (mmHg). Most scholars agree that on average, “normal pressure” should be between 5-15 mmHg and that 20-25 mmHg is when the ICP crosses the line into being IH. Pressure can be brought on by several different means: space-occupying masses such as hydrocephalus and cranial cysts/tumors; cranial edema (Encephalitis); trauma; stroke; aneurysm; certain infections/diseases (Meningitis), liver failure
Intracranial Hypertension (IH) means high pressure inside the skull. Intracranial Pressure (ICP) is measured in millimeters of mercury (mmHg). Most scholars agree that on average, “normal pressure” should be between 5-15 mmHg and that 20-25 mmHg is when the ICP crosses the line into being IH. Pressure can be brought on by several different means: space-occupying masses such as hydrocephalus and cranial cysts/tumors; cranial edema (Encephalitis); trauma; stroke; aneurysm; certain infections/diseases (Meningitis), liver failure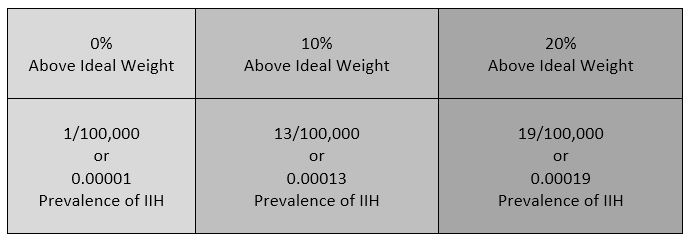 Additionally, the weight factor excludes men and children under the age of 10, which may simply be because women are more likely than men to have comorbid conditions that would lead to Intracranial Hypertension. Studies show that the women to men ratio for Chiari Malformation is believed to be 3:1 and those with both Chiari Malformation and Ehlers-Danlos Syndromes 9:1
Additionally, the weight factor excludes men and children under the age of 10, which may simply be because women are more likely than men to have comorbid conditions that would lead to Intracranial Hypertension. Studies show that the women to men ratio for Chiari Malformation is believed to be 3:1 and those with both Chiari Malformation and Ehlers-Danlos Syndromes 9:1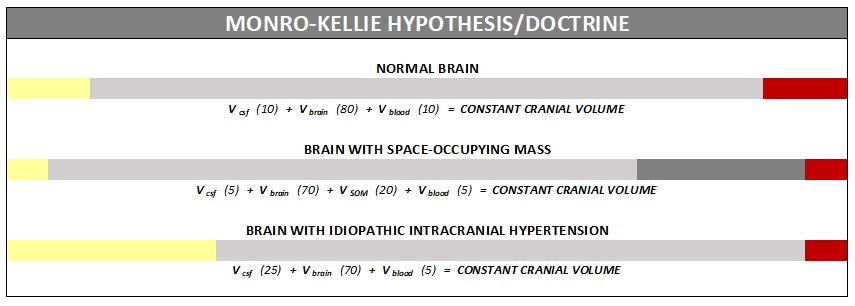
 Diagnosis of Intracranial Hypertension usually begins with investigating either the headaches or the vision problems. The least invasive test is having a neuro-ophthalmologist check behind your eyes for Papilledema. It is not considered conclusive in testing for IH, but it is essential in determining the extent of the damage to the optical nerves. Magnetic Resonance Imaging (MRI) of the brain can be useful in showing signs of Intracranial Hypertension. In cases where one or more space-occupying masses exists, further imaging and often biopsy may be required. The type of mass, its exact location, and the amount of damage that it is believed to be doing, will be used to determine the best treatment. If imaging gives an indication that the intracranial pressure is high, but no space-occupying mass exists, additional testing is usually necessary to confirm, some of which can be potentially be dangerous for those with Heritable Disorders of Connective Tissue (HDCT), such as Ehlers-Danlos Syndromes (EDS). Lumbar punctures (LP), also known as a spinal tap, are often used to test the
Diagnosis of Intracranial Hypertension usually begins with investigating either the headaches or the vision problems. The least invasive test is having a neuro-ophthalmologist check behind your eyes for Papilledema. It is not considered conclusive in testing for IH, but it is essential in determining the extent of the damage to the optical nerves. Magnetic Resonance Imaging (MRI) of the brain can be useful in showing signs of Intracranial Hypertension. In cases where one or more space-occupying masses exists, further imaging and often biopsy may be required. The type of mass, its exact location, and the amount of damage that it is believed to be doing, will be used to determine the best treatment. If imaging gives an indication that the intracranial pressure is high, but no space-occupying mass exists, additional testing is usually necessary to confirm, some of which can be potentially be dangerous for those with Heritable Disorders of Connective Tissue (HDCT), such as Ehlers-Danlos Syndromes (EDS). Lumbar punctures (LP), also known as a spinal tap, are often used to test the 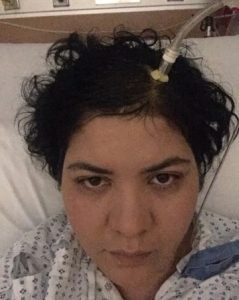 opening CSF pressure, but by puncturing the dura (which is thinner than normal with Connective Tissue Disorders), the risk of a CSF leak is high. When an LP causes a CSF leak, the first indication is usually a post-dural-puncture headache (PLPH) and eventually, the intracranial hypertension will decrease, as the leak causes intracranial hypotension.
opening CSF pressure, but by puncturing the dura (which is thinner than normal with Connective Tissue Disorders), the risk of a CSF leak is high. When an LP causes a CSF leak, the first indication is usually a post-dural-puncture headache (PLPH) and eventually, the intracranial hypertension will decrease, as the leak causes intracranial hypotension.

 But to look at the full history of what became known as a Chiari Malformation, we can begin by looking at the research of a German pathologist, named Theodor Langhans. In his research in 1881 (a decade before Hans Chiari conducted his research on what became known as a Chiari Malformation), while looking at syringomyelia (“a cavity created in the spinal cord”), he noted a “change in the cerebellar cavity.” Upon dissection of the cerebellum, he described the cerebellar tonsils as “two symmetrical pyramidal tumors,” pushing the brainstem forward.
But to look at the full history of what became known as a Chiari Malformation, we can begin by looking at the research of a German pathologist, named Theodor Langhans. In his research in 1881 (a decade before Hans Chiari conducted his research on what became known as a Chiari Malformation), while looking at syringomyelia (“a cavity created in the spinal cord”), he noted a “change in the cerebellar cavity.” Upon dissection of the cerebellum, he described the cerebellar tonsils as “two symmetrical pyramidal tumors,” pushing the brainstem forward. Unfortunately it leaves most of us with failed decompressions, fighting with our neurosurgeons that “something is still wrong.” These neurosurgeons look at their post-operative checklist and see that they successfully did everything surgically required in their out-of-date textbooks:
Unfortunately it leaves most of us with failed decompressions, fighting with our neurosurgeons that “something is still wrong.” These neurosurgeons look at their post-operative checklist and see that they successfully did everything surgically required in their out-of-date textbooks: