INTRACRANIAL HYPERTENSION (IH) AND IDIOPATHIC INTRACRANIAL HYPERTENSION (IIH) ARE CONNECTED, BUT ARE NOT THE SAME THING AND THEREFORE SHOULD NOT BE USED INTERCHANGEABLY.
 Intracranial Hypertension (IH) means high pressure inside the skull. Intracranial Pressure (ICP) is measured in millimeters of mercury (mmHg). Most scholars agree that on average, “normal pressure” should be between 5-15 mmHg and that 20-25 mmHg is when the ICP crosses the line into being IH. Pressure can be brought on by several different means: space-occupying masses such as hydrocephalus and cranial cysts/tumors; cranial edema (Encephalitis); trauma; stroke; aneurysm; certain infections/diseases (Meningitis), liver failure[1], kidney failure[2]; or as a side-effect of certain medications (such as: Tetracycline[3][5], Sulfasalazine[4], Lithium[5], excess amounts of Vitamin A, steroid use[6], growth hormone treatments[6], and the hormonal Intrauterine Device (IUD), “Mirena”[7]); however, sometimes the cause of the pressure is completely unknown. When an etiological cofactor exists, it is considered Secondary Intracranial Hypertension (SIH); when no other cause is identified, it is known as Idiopathic Intracranial Hypertension (IIH) or Primary Intracranial Hypertension (PIH).
Intracranial Hypertension (IH) means high pressure inside the skull. Intracranial Pressure (ICP) is measured in millimeters of mercury (mmHg). Most scholars agree that on average, “normal pressure” should be between 5-15 mmHg and that 20-25 mmHg is when the ICP crosses the line into being IH. Pressure can be brought on by several different means: space-occupying masses such as hydrocephalus and cranial cysts/tumors; cranial edema (Encephalitis); trauma; stroke; aneurysm; certain infections/diseases (Meningitis), liver failure[1], kidney failure[2]; or as a side-effect of certain medications (such as: Tetracycline[3][5], Sulfasalazine[4], Lithium[5], excess amounts of Vitamin A, steroid use[6], growth hormone treatments[6], and the hormonal Intrauterine Device (IUD), “Mirena”[7]); however, sometimes the cause of the pressure is completely unknown. When an etiological cofactor exists, it is considered Secondary Intracranial Hypertension (SIH); when no other cause is identified, it is known as Idiopathic Intracranial Hypertension (IIH) or Primary Intracranial Hypertension (PIH).
“Idiopathic Intracranial Hypertension (IIH) was first noticed in 1893, by the German physician Heinrich Quincke, who named it Serous Meningitis. As its absence of space occupying masses/lesions began to draw more thought, it was renamed Pseudotumor Cerebri (PTC) by Max Nonne in 1904. Sometime later, the term “Benign Intracranial Hypertension” began being used interchangeably with Pseudotumor Cerebri, to describe the fact that while it is sharing some of the same characteristics that a cranial tumor would cause, it is benign (not harmful), but arguments were made against it in that blindness is not indicative of being benign.”[6] The name finally settled as “Idiopathic Intracranial Hypertension,” which means IH of an unknown cause. No matter what you choose to call it, the pain and damage remains the same for those who have it.
UNDERSTANDING IDIOPATHIC INTRACRANIAL HYPERTENSION
IIH is a neurological disorder where the cerebrospinal fluid within the skull is elevated, without the presence of a space-occupying mass, edema (brought on by things such as trauma, infection, or disease), or any adverse reactions to certain medications. Studies show that IIH is more common amongst women between the ages of 20 and 50,[8] and there is a slight increase amongst those that are overweight. Some studies also suggest a connection between obstructive sleep apnea and transverse cerebral venous sinus stenosis.[9] Amongst the general population, IIH is believed to exist in 1/100,000 (0.00001). Amongst those that are 10% above their ideal body weight, the numbers increase to 13/100,000 (0.00013), and rising to 19/100,000 (0.00019) in those 20% above their ideal body weight.[10] Although doctors often tend to pass this off as merely a side effect of weight gain, the increase is slim and seems to decrease as the percentage of weight gain above ideal weight continues to rise above the 10% margin. 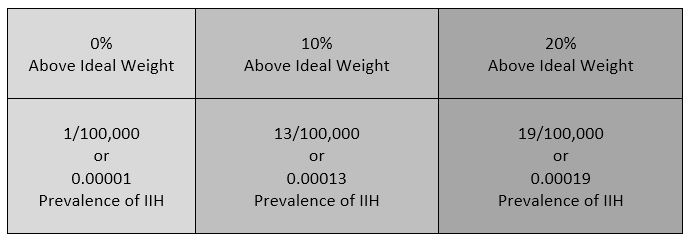 Additionally, the weight factor excludes men and children under the age of 10, which may simply be because women are more likely than men to have comorbid conditions that would lead to Intracranial Hypertension. Studies show that the women to men ratio for Chiari Malformation is believed to be 3:1 and those with both Chiari Malformation and Ehlers-Danlos Syndromes 9:1[11]). However weight is not irrelevant with IIH, the overweight/obese patient population report finding improvement of some symptoms when weight loss of 5-10% of one’s overall body weight, when accompanied by a low-salt diet[12].
Additionally, the weight factor excludes men and children under the age of 10, which may simply be because women are more likely than men to have comorbid conditions that would lead to Intracranial Hypertension. Studies show that the women to men ratio for Chiari Malformation is believed to be 3:1 and those with both Chiari Malformation and Ehlers-Danlos Syndromes 9:1[11]). However weight is not irrelevant with IIH, the overweight/obese patient population report finding improvement of some symptoms when weight loss of 5-10% of one’s overall body weight, when accompanied by a low-salt diet[12].
UNDERSTANDING THE IH/IIH CONNECTION: THE MONRO-KELLIE DOCTRINE
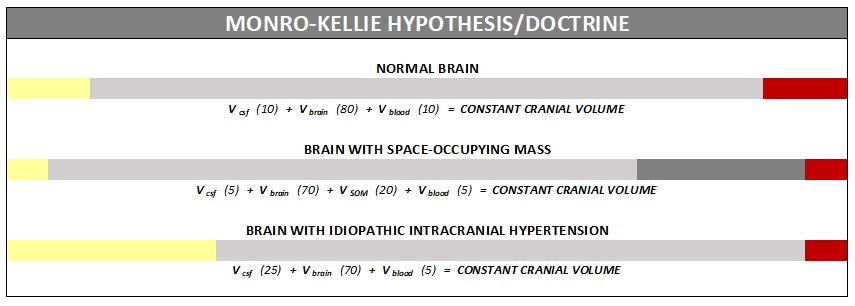
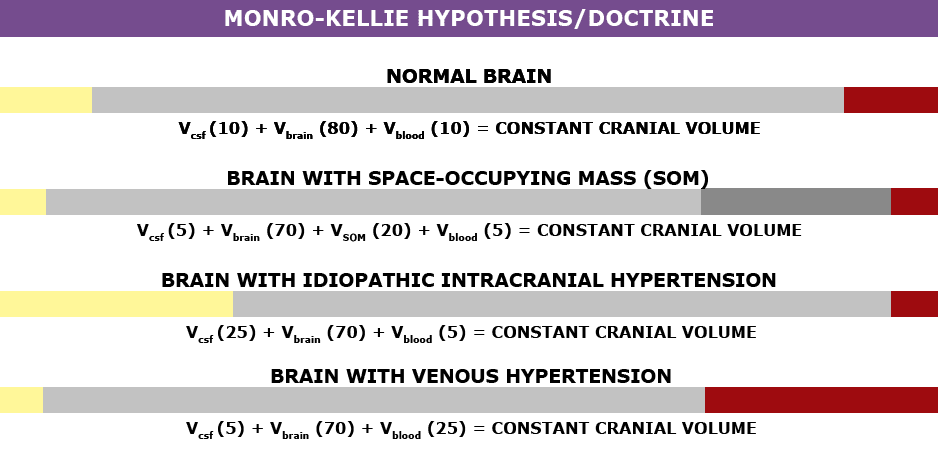
The association between IH/IIH and Chiari Malformation, appears to be a malicious intricate pathological circle. The cranium (skull) consists of brain matter, cerebrospinal fluid, and both venous and arterial blood. A hypothesis, referred to as the Monro-Kellie Hypothesis (or Monro-Kellie Doctrine), states, “The sum of volumes of brain, CSF, and intracranial blood is constant. An increase in one should cause a decrease in one or both of the remaining two.”[13] Therefore, if there is an abundance of cerebrospinal fluid (IIH or hydrocephalus), both cranial blood volume and brain matter should be forced to deplete. This depletion is usually directed in the path of least resistance – through the foramen magnum and into the spinal canal. When the cranial brain matter closest to the bottom of the skull (cerebellar tonsils) goes through the foramen magnum and into the spinal canal (an Acquired Chiari Malformation), it blocks the flow of cerebrospinal fluid, which in turn, continues to raise intracranial pressure.
SYMPTOMS OF INTRACRANIAL HYPERTENSION
Intracranial Hypertension (IH) can be either acute or chronic and comes with a variety of symptoms, many of which can help distinguish IH pain from typical pain associated with Chiari Malformation. A typical Chiari headache originates at the back of the skull (at the occiput), but IH headaches are usually described as pressure at the top of the head, that radiates downward. Headaches tend to be worse when laying down (which is opposite of low pressure headaches that are often relieved by laying down). Those that suffer from IH, often report waking up from sleep with a bad headache, and often a slight incline can help alleviate the headache pain. Pulsatile Tinnitus occurs when you hear a ringing in your ears that coincides with your heart beat. The tale-tell symptom of IH involves the damage done to the optical nerves. Papilledema is when the optic discs swell in response to the increased cranial pressure.[14] Symptoms of Papilledema include: headaches behind the eyes, blurred vision, fleeting vision, dimmed vision, double vision, visual obscurations, decreased peripheral vision, and photopsia. Another source of IH damage is seen in the pituitary gland and is known as Empty Sella Syndrome (ESS). As the high intracranial pressure (ICP) tries to take over, cerebrospinal fluid finds its way to the sella turcica and starts filling it with spinal fluid (partially or completely)[15]. The intruding CSF attempts to envelope this depression in the sphenoid bone, and squeezes the pituitary gland, flattening it until it appears “empty.” While some initially suffer no symptoms of the damage done to the pituitary gland, most eventually develop a variety of hormonal issues, known as hypopituitarism.
DIAGNOSIS CRITERIA
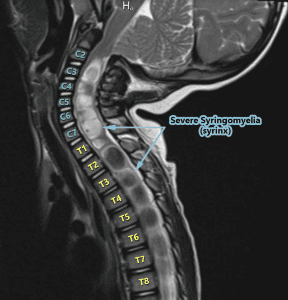
 Diagnosis of Intracranial Hypertension usually begins with investigating either the headaches or the vision problems. The least invasive test is having a neuro-ophthalmologist check behind your eyes for Papilledema. It is not considered conclusive in testing for IH, but it is essential in determining the extent of the damage to the optical nerves. Magnetic Resonance Imaging (MRI) of the brain can be useful in showing signs of Intracranial Hypertension. In cases where one or more space-occupying masses exists, further imaging and often biopsy may be required. The type of mass, its exact location, and the amount of damage that it is believed to be doing, will be used to determine the best treatment. If imaging gives an indication that the intracranial pressure is high, but no space-occupying mass exists, additional testing is usually necessary to confirm, some of which can be potentially be dangerous for those with Heritable Disorders of Connective Tissue (HDCT), such as Ehlers-Danlos Syndromes (EDS). Lumbar punctures (LP), also known as a spinal tap, are often used to test the
Diagnosis of Intracranial Hypertension usually begins with investigating either the headaches or the vision problems. The least invasive test is having a neuro-ophthalmologist check behind your eyes for Papilledema. It is not considered conclusive in testing for IH, but it is essential in determining the extent of the damage to the optical nerves. Magnetic Resonance Imaging (MRI) of the brain can be useful in showing signs of Intracranial Hypertension. In cases where one or more space-occupying masses exists, further imaging and often biopsy may be required. The type of mass, its exact location, and the amount of damage that it is believed to be doing, will be used to determine the best treatment. If imaging gives an indication that the intracranial pressure is high, but no space-occupying mass exists, additional testing is usually necessary to confirm, some of which can be potentially be dangerous for those with Heritable Disorders of Connective Tissue (HDCT), such as Ehlers-Danlos Syndromes (EDS). Lumbar punctures (LP), also known as a spinal tap, are often used to test the  opening CSF pressure, but by puncturing the dura (which is thinner than normal with Connective Tissue Disorders), the risk of a CSF leak is high. When an LP causes a CSF leak, the first indication is usually a post-dural-puncture headache (PLPH) and eventually, the intracranial hypertension will decrease, as the leak causes intracranial hypotension.[16] CSF leaks can escalate very quickly and can be difficult to identify and treat; therefore, we recommend that LPs be done only when absolutely necessary, and that they be done only under fluoroscopy, by qualified surgeons that fully understand the likelihood of Connective Tissue Disorders, the symptoms of leaks, and have a plan of action should those symptoms occur. Sometimes, ICP can fluctuate and have high spikes that cause problems, rendering LPs useless unless they are done at the precise time. When these spikes are suspected ICP monitoring bolts might be the better option, but still poses a risk of leaks.[17]
opening CSF pressure, but by puncturing the dura (which is thinner than normal with Connective Tissue Disorders), the risk of a CSF leak is high. When an LP causes a CSF leak, the first indication is usually a post-dural-puncture headache (PLPH) and eventually, the intracranial hypertension will decrease, as the leak causes intracranial hypotension.[16] CSF leaks can escalate very quickly and can be difficult to identify and treat; therefore, we recommend that LPs be done only when absolutely necessary, and that they be done only under fluoroscopy, by qualified surgeons that fully understand the likelihood of Connective Tissue Disorders, the symptoms of leaks, and have a plan of action should those symptoms occur. Sometimes, ICP can fluctuate and have high spikes that cause problems, rendering LPs useless unless they are done at the precise time. When these spikes are suspected ICP monitoring bolts might be the better option, but still poses a risk of leaks.[17]
TRANSVERSE SINUS STENOSIS (TSS)
Transverse sinus stenosis (TSS) occurs when there is a narrowing of the transverse sinus (dural venous sinus), which in turn can compromise cerebral venous outflow. TSS is common in idiopathic intracranial hypertension (IIH). Depending on the study that you are reading, it is proving to be present in 65-100% of those diagnosed specifically with IIH. Its direct connection seems relatively obscure, and there is no indication of its prevalence in intracranial hypertension (IH), but it is worth looking for and treating if found. While scholars remain undecided as to whether TSS is a cause or consequence of IH, if it does prove to be a cause of high pressure, IIH will likely no longer have an idiopathic element to it and it will become another etiology of Intracranial Hypertension. TSS can often be undetectable with standard Magnetic Resonance Imaging (MRI). The correct procedure would be Magnetic Resonance Venography (MRV, with the ATECO technique [18]), specifically looking for signs of stenosis, to include looking for fistula(s) and aneurysm(s). The lack of a fistula or aneurysms however, does not exclude the possibility of a TSS existing (remember it’s being found in 65-100% of those with IIH). Even with MRV, TSS can often be misinterpreted as “flow-related artifacts.” [18] Because the prevalence of TSS in IIH patients is high (some studies call it “universal”) [19], we recommend that all IIH patients have a MRV with the ATECO technique done before surgical treatment and that venous stenting be considered as a viable surgical treatment.
TREATMENT OPTIONS
Treatments for Idiopathic Intracranial Hypertension usually starts with weight loss and/or medicinal options; Diamox (Acetazolamide) and Topamax (Topiramate) are most frequently prescribed. Those with IH/IIH should avoid consuming caffeine, as it can increase pressure and therefore is counter-productive to treatment measures. Diamox is a carbonic anhydrase inhibitor and Topamax can also inhibit carbonic anhydrase, but is an anticonvulsant, often prescribed for the treatment of neuropathy and seizure disorders. Both are believed to successfully lower the production of cerebrospinal fluid. Topamax can also help suppress the appetite, which can help with weight loss, but it also comes with many side-effects like all nerve meds do. When medication fails to decrease ICP, a Ventriculoperitoneal Shunt (VP Shunt) or Ventriculoatrial Shunt (VA Shunt) are surgically placed to drain cerebrospinal fluid straight from the ventricle. Shunts are known for failing and often need a multitude of revisions. Venous stenting is not a new procedure, yet it is not readily offered. While there are studies indicating that the successful reduction of intracranial pressure can help with TSS. Stenting is not only a surgical treatment for the stenosis (which could significantly reduce the possibility of a life-threatening aneurysm in patients with a connective tissue disorder), but it is also a surgical treatment for intracranial hypertension as it “improves CSF resorption in the venous system.” [18] Therefore, it seems illogical to shunt (just dealing with the pressure) and leave such a potentially life-threatening condition untreated. [20] Studies are indicating as high as a 94% of patients being cured of all IIH symptoms as a direct result of venous stenting. [18] While all surgeries pose a risk of complications, and the statistics for stenting are likely inflated and skewed (like that of decompression surgeries), these statistics on stenting are definitely encouraging!
Intracranial Hypertension is a complex issue that should be explored whenever a Chiari Malformation exists, before a decompression surgery is performed. When both Intracranial Hypertension and Chiari Malformation are found to co-exist, the treatment should be in consideration of the correlation of the two, as they both are pathological co-factors of one another. Failure to recognize and treat Intracranial Hypertension before or soon after decompression surgery, will increase the likelihood of a failed decompression. While a decompression surgery can lower Intracranial Hypertension, as cerebrospinal fluid is once again allowed to flow, if space-occupying masses or a case of Idiopathic Intracranial Hypertension (where too much cerebrospinal fluid is being created) are left untreated, those problems will still exist after decompression surgery and the high pressure is likely to cause the cerebellar tonsils to fall once again.
*Revised October 2018
[wpedon id=”4396″ align=”center”]
References:
1 Jalan, R. “Intracranial Hypertension in Acute Liver Failure: Pathophysiological Basis of Rational Management.” Seminars in Liver Disease., U.S. National Library of Medicine, Aug. 2003, <www.ncbi.nlm.nih.gov/pubmed/14523680>.
2 Chang, D, et al. “Benign Intracranial Hypertension and Chronic Renal Failure.” Cleveland Clinic Journal of Medicine., U.S. National Library of Medicine, <www.ncbi.nlm.nih.gov/pubmed/1525975>.
3 Holst, Anders Vedel, et al. “A Severe Case of Tetracycline-Induced Intracranial Hypertension.”Dermatology Reports, PAGEPress Publications, 31 Jan. 2011, <www.ncbi.nlm.nih.gov/pmc/articles/PMC4211491/>.
4 Sevgi, E, et al. “Drug Induced Intracranial Hypertension Associated with Sulphasalazine Treatment.” Headache., U.S. National Library of Medicine, Feb. 2008, <www.ncbi.nlm.nih.gov/pubmed/18070060>.
5 Kelly, S J, et al. “Pseudotumor Cerebri Associated with Lithium Use in an 11-Year-Old Boy.”Journal of AAPOS : the Official Publication of the American Association for Pediatric Ophthalmology and Strabismus., U.S. National Library of Medicine, Apr. 2009, <www.ncbi.nlm.nih.gov/pubmed/19393521>.
6 Aylward, Shawn C. “Intracranial Hypertension: Is It Primary, Secondary, or Idiopathic?”Journal of Neurosciences in Rural Practice, Medknow Publications & Media Pvt Ltd, 2014, <www.ncbi.nlm.nih.gov/pmc/articles/PMC4173226/>.
7 Etminan, Mahyar, et al. “Risk of Intracranial Hypertension with Intrauterine Levonorgestrel.”Therapeutic Advances in Drug Safety, SAGE Publications, June 2015, <www.ncbi.nlm.nih.gov/pmc/articles/PMC4519742/>.
8 “Pseudotumor Cerebri Information Page.” National Institute of Neurological Disorders and Stroke, U.S. Department of Health and Human Services, <www.ninds.nih.gov/Disorders/All-Disorders/Pseudotumor-Cerebri-Information-Page>.
9 Thurtell, Matthew J., et al. “An Update on Idiopathic Intracranial Hypertension.” Reviews in Neurological Diseases, U.S. National Library of Medicine, 2010, <www.ncbi.nlm.nih.gov/pmc/articles/PMC3674489/>.
10 Wani, Irfan Yousuf, et al. “Complete Ophthalmoplegia: A Rare Presentation of Idiopathic Intracranial Hypertension.” Annals of Indian Academy of Neurology, Medknow Publications & Media Pvt Ltd, 2015, <www.ncbi.nlm.nih.gov/pmc/articles/PMC4683894/>.
11 Henderson, Fraser C., et al. “Neurological and Spinal Manifestations of the Ehlers–Danlos Syndromes.” American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 21 Feb. 2017, <www.onlinelibrary.wiley.com/doi/10.1002/ajmg.c.31549/full>.
12 Thurtell, Matthew J., and Michael Wall. “Idiopathic Intracranial Hypertension (Pseudotumor Cerebri): Recognition, Treatment, and Ongoing Management.” Current Treatment Options in Neurology, U.S. National Library of Medicine, Feb. 2013,<www.ncbi.nlm.nih.gov/pmc/articles/PMC3554852/>.
13 Mokri, B. “The Monro-Kellie Hypothesis: Applications in CSF Volume Depletion.” Neurology., U.S. National Library of Medicine, 26 June 2001, <www.ncbi.nlm.nih.gov/pubmed/11425944>.
14 Schirmer, Clemens M, and Thomas R Hedges. “Mechanisms of Visual Loss in Papilledema.”Journal of Neurosurgery, <www.thejns.org/doi/full/10.3171/FOC-07/11/E5>.
15 “Empty Sella Syndrome Information Page.” National Institute of Neurological Disorders and Stroke, U.S. Department of Health and Human Services, <www.ninds.nih.gov/Disorders/All-Disorders/Empty-Sella-Syndrome-Information-Page>.
16 Panikkath, Ragesh, et al. “Intracranial Hypertension and Intracranial Hypotension Causing Headache in the Same Patient.” Proceedings (Baylor University. Medical Center), Baylor Health Care System, July 2014, <www.ncbi.nlm.nih.gov/pmc/articles/PMC4059569/>.
17 Abraham, Mary, and Vasudha Singhal. “Intracranial Pressure Monitoring.” Journal of Neuroanaesthesiology, <www.jnaccjournal.org/article.asp?issn=2348-0548;year=2015;volume=2;issue=3;spage=193;epage=203;aulast=Abraham>.
18 Ahmed, Wilkinson, et al. “Transverse Sinus Stenting for Idiopathic Intracranial Hypertension: A Review of 52 Patients and of Model Prediction.” American Society of Neuroradiology, July 2011. <www.ajnr.org/content/32/8/1408.long>.
19 Riggeal, Bruce, et al. “Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis.” American Academy of Neurology, 2012. <www.ncbi.nlm.nih.gov/pmc/articles/PMC3589184/>.
20 Patel, et al. “Evaluating and treating venous outflow stenoses is necessary for the successful open surgical treatment of arteriovenous fistula aneurysms.” Society for Clinical Vascular Surgery, Volume 61, Issue 2. February 2015. <www.sciencedirect.com/science/article/pii/S0741521414014116>.



![Brain Under Pressure – Understanding Intracranial Hypertension [UPDATED]](https://chiaribridges.org/wp-content/uploads/2021/06/IH_AS271511956_resized.jpg)


![The Important Questions to Ask Your Neurosurgeon [Revised]](https://chiaribridges.org/wp-content/uploads/2023/09/MRI-doctor_AS505903501.jpg)








![Brain Under Pressure – Understanding Intracranial Hypertension [Archived]](https://chiaribridges.org/wp-content/uploads/2017/12/Fotolia_87985277_M.jpg)
 Intracranial Hypertension (IH) means high pressure inside the skull. Intracranial Pressure (ICP) is measured in millimeters of mercury (mmHg). Most scholars agree that on average, “normal pressure” should be between 5-15 mmHg and that 20-25 mmHg is when the ICP crosses the line into being IH. Pressure can be brought on by several different means: space-occupying masses such as hydrocephalus and cranial cysts/tumors; cranial edema (Encephalitis); trauma; stroke; aneurysm; certain infections/diseases (Meningitis), liver failure
Intracranial Hypertension (IH) means high pressure inside the skull. Intracranial Pressure (ICP) is measured in millimeters of mercury (mmHg). Most scholars agree that on average, “normal pressure” should be between 5-15 mmHg and that 20-25 mmHg is when the ICP crosses the line into being IH. Pressure can be brought on by several different means: space-occupying masses such as hydrocephalus and cranial cysts/tumors; cranial edema (Encephalitis); trauma; stroke; aneurysm; certain infections/diseases (Meningitis), liver failure Additionally, the weight factor excludes men and children under the age of 10, which may simply be because women are more likely than men to have comorbid conditions that would lead to Intracranial Hypertension. Studies show that the women to men ratio for Chiari Malformation is believed to be 3:1 and those with both Chiari Malformation and Ehlers-Danlos Syndromes 9:1
Additionally, the weight factor excludes men and children under the age of 10, which may simply be because women are more likely than men to have comorbid conditions that would lead to Intracranial Hypertension. Studies show that the women to men ratio for Chiari Malformation is believed to be 3:1 and those with both Chiari Malformation and Ehlers-Danlos Syndromes 9:1
 Diagnosis of Intracranial Hypertension usually begins with investigating either the headaches or the vision problems. The least invasive test is having a neuro-ophthalmologist check behind your eyes for Papilledema. It is not considered conclusive in testing for IH, but it is essential in determining the extent of the damage to the optical nerves. Magnetic Resonance Imaging (MRI) of the brain can be useful in showing signs of Intracranial Hypertension. In cases where one or more space-occupying masses exists, further imaging and often biopsy may be required. The type of mass, its exact location, and the amount of damage that it is believed to be doing, will be used to determine the best treatment. If imaging gives an indication that the intracranial pressure is high, but no space-occupying mass exists, additional testing is usually necessary to confirm, some of which can be potentially be dangerous for those with Heritable Disorders of Connective Tissue (HDCT), such as Ehlers-Danlos Syndromes (EDS). Lumbar punctures (LP), also known as a spinal tap, are often used to test the
Diagnosis of Intracranial Hypertension usually begins with investigating either the headaches or the vision problems. The least invasive test is having a neuro-ophthalmologist check behind your eyes for Papilledema. It is not considered conclusive in testing for IH, but it is essential in determining the extent of the damage to the optical nerves. Magnetic Resonance Imaging (MRI) of the brain can be useful in showing signs of Intracranial Hypertension. In cases where one or more space-occupying masses exists, further imaging and often biopsy may be required. The type of mass, its exact location, and the amount of damage that it is believed to be doing, will be used to determine the best treatment. If imaging gives an indication that the intracranial pressure is high, but no space-occupying mass exists, additional testing is usually necessary to confirm, some of which can be potentially be dangerous for those with Heritable Disorders of Connective Tissue (HDCT), such as Ehlers-Danlos Syndromes (EDS). Lumbar punctures (LP), also known as a spinal tap, are often used to test the  opening CSF pressure, but by puncturing the dura (which is thinner than normal with Connective Tissue Disorders), the risk of a CSF leak is high. When an LP causes a CSF leak, the first indication is usually a post-dural-puncture headache (PLPH) and eventually, the intracranial hypertension will decrease, as the leak causes intracranial hypotension.
opening CSF pressure, but by puncturing the dura (which is thinner than normal with Connective Tissue Disorders), the risk of a CSF leak is high. When an LP causes a CSF leak, the first indication is usually a post-dural-puncture headache (PLPH) and eventually, the intracranial hypertension will decrease, as the leak causes intracranial hypotension.